Blog
3 Trends Reshaping Pharmacy Benefits Management
Recent trends in pharmacy benefits, including the rise of GLP-1 medications and increased pharmacy benefits management (PBM) scrutiny, are reshaping employer healthcare costs and employee wellness strategies, making it crucial for benefits professionals to stay informed and adapt their programs accordingly.
In this blog and the webinar linked below, we offer a comprehensive analysis of three critical areas transforming the sector: GLP-1 medications, biosimilars, and PBM transparency. Our analysis covers diabetes and weight loss management trends, the impact of biosimilars on the market, and the complexities of PBM rebate structures.
This overview equips employers with actionable insights that will enable strategic decision-making in benefits programs, ultimately optimizing both employee health outcomes and financial performance. For more in-depth analysis, view the full webinar recording below.
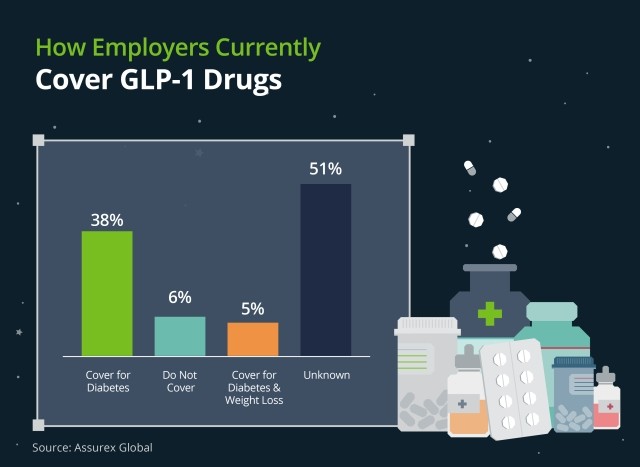
GLP-1s: A Weighty Decision
The prevalence of diabetes in the United States has reached alarming levels, with approximately 12% of adults diagnosed with the condition. Even more concerning is the additional 40% of the population classified as pre-diabetic, underscoring the potential for a significant increase in diabetes diagnoses in the near future. This epidemiological landscape has profound implications for employer-sponsored health plans, particularly in relation to the coverage of GLP-1 (Glucagon-Like Peptide-1) receptor agonists.
GLP-1 receptor agonists have garnered substantial attention due to their dual approved indications: diabetes management and weight loss. The efficacy of these medications in addressing both conditions has led to a surge in demand, presenting a complex decision-making scenario for employers. On one hand, the potential for improved health outcomes and reduced long-term complications associated with diabetes and obesity is compelling. Conversely, the high cost of these medications poses a significant financial burden on health plans.
Employers must carefully weigh the short-term financial impact against the potential long-term benefits of covering GLP-1 medications. This decision requires a nuanced understanding of plan demographics, overall health trends within the employee population, and the organization's financial capacity. Moreover, employers should consider implementing comprehensive wellness programs and diabetes management initiatives to complement pharmaceutical interventions, potentially mitigating the need for costly medications in some cases.
The State of Biosimilars
The landscape of biosimilars is poised for rapid evolution, with accelerated adoption rates anticipated in the coming year. Industry experts project that the traditional six- to nine-month lag in PBM incorporation of biosimilars may be significantly shortened, driven primarily by the actions of major PBMs. Notably, there is speculation that CVS Caremark (owner of Cordavis) may lead this charge by preferring a Cordavis-branded Stelara biosimilar as early as January 1st, potentially utilizing a rebate credit mechanism to incentivize adoption. Both Express Scripts and OptumRx have sibling companies that “private label” biosimilars with preferred placements on their formularies. The question on the mind of plan sponsors and advisors is, “Why is PBM putting a drug on their formulary that their firm makes money on?”
This shift in biosimilar adoption strategies has profound implications for employer benefit plans, particularly in terms of formulary composition and rebate structures. The potential for sudden formulary changes, typically announced quarterly, necessitates heightened vigilance from employers and their benefits consultant. Of particular concern is the potential impact on rebate yields, which many organizations rely on for various purposes within their benefits programs.
To navigate this dynamic environment, employers must adopt a proactive stance in their benefit planning processes. This includes maintaining open lines of communication with PBMs and benefits consultants, conducting regular reviews of formulary changes, and modeling various scenarios to assess the financial impact of biosimilar adoption.
Furthermore, employers should consider incorporating flexibility into their PBM contracts to accommodate rapid shifts in the biosimilar landscape, ensuring their ability to leverage cost-saving opportunities as they arise.
Impact of PBM Distrust
The pharmacy benefits management industry has long been subject to scrutiny, but recent years have witnessed an unprecedented surge in distrust. This escalating skepticism is rooted in concerns over transparency, pricing practices, and potential conflicts of interest within the PBM business model. The amplification of these concerns has been further exacerbated by high-profile critiques and investigations, catalyzing a shift in employer attitudes towards traditional PBM arrangements.
In response to this growing distrust, a notable trend has emerged: Employers are increasingly exploring alternatives to the "Big 3" PBMs, gravitating towards smaller, more transparent PBM models. These alternative PBMs often offer enhanced visibility into pricing mechanisms, more flexible contract terms, and a greater alignment of interests with plan sponsors. This shift represents a fundamental reevaluation of the PBM-employer relationship, with a heightened emphasis on accountability and value demonstration.
The implications of this trend extend beyond mere vendor selection. Employers are now recognizing the critical importance of their benefits consultant conducting thorough analyses of PBM contract language and plan structure. This heightened scrutiny necessitates a more sophisticated approach to benefits management, often requiring specialized expertise to navigate the intricate nuances of PBM agreements and operations.
Next Steps for Employers
- Ask your benefits consultant to conduct a comprehensive review of current PBM arrangements, focusing on transparency, rebate structures, and formulary management practices.
- Engage with your benefits consultant to explore alternative PBM models, including smaller, more transparent options that align with organizational objectives.
- Develop a strategic approach to GLP-1 coverage, considering both the potential health benefits and financial implications for the organization.
- Implement robust monitoring systems to track biosimilar adoption and its impact on plan costs and rebate structures.
- Invest in employee education initiatives to promote understanding of benefit changes and encourage informed healthcare decision-making.
The rapidly evolving landscape of pharmacy benefits management presents both challenges and opportunities for employers. By adopting a proactive, analytical approach to PBM relationships, biosimilar adoption, and emerging therapeutic options like GLP-1s, organizations can optimize their benefits strategies to better serve their employees while managing costs effectively. As the industry continues to transform, staying informed and agile in decision-making will be crucial for navigating the complex intersection of healthcare innovation and employee benefits management.
Our Mission to More series offers guidance from leading specialists on what employees want and how employers can adapt to the new benefits universe. For more guidance on the latest in employee benefits, sign up for Woodruff Sawyer’s Benefits newsletter, which includes all Mission to More articles.

Author
Table of Contents










